1. Atomic design of metal-based catalysts for HOR
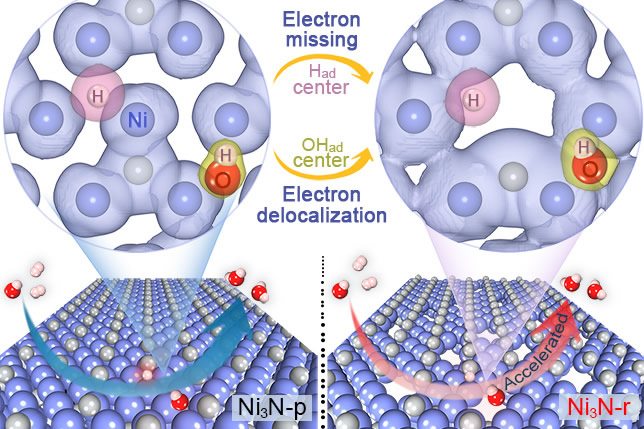
Anion exchange membrane fuel cells are highly attractive as an efficient energy conversion device owing to the advantages of employing economic catalysts in alkaline electrolytes. However, the kinetics of the anodic hydrogen oxidation reaction (HOR) over catalysts becomes relatively sluggish in alkaline electrolytes in comparation with that in acid systems, due to the mismatched adsorption behaviors during HOR. To this end, we focus on the atomic design of metal-based catalysts with well-tuned adsorption behaviors for HOR. Recent papers are listed as follows:
2. Bond design of metal-based catalysts for OER
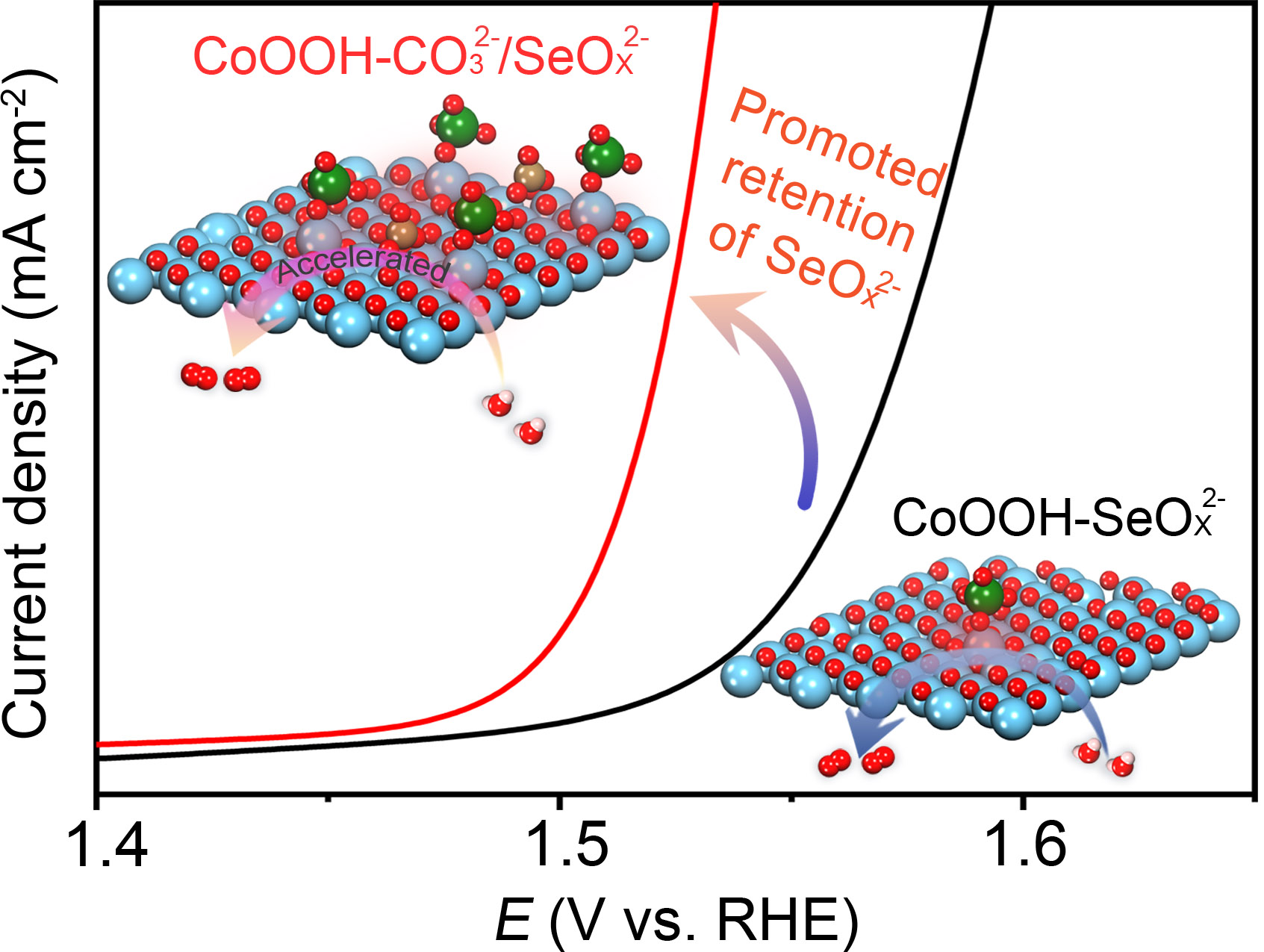
Transition metal-based electrocatalysts for practical oxygen evolution reaction (OER) usually undergo ambiguous and restricted reaction pathways with unsatisfied performance due to the uncontrolled bond coordination at metal active sites. Understanding and further regulating the bond coordination of metal active sites under catalytic OER condition is of significant importance to develop highly efficient and stable non-noble metal-based catalysts for practical water electrolysis. We focus on that issue and keep making progress. Recent papers are listed as follows:
1. Engineering the electrical conductivity of lamellar silver-doped cobalt(II) selenide nanobelts for enhanced oxygen evolution. Angew. Chem. Int. Ed. 2017, 56, 328.
2. Isolated Pd atom anchoring endows cobalt diselenides with regulated water-reduction kinetics for alkaline hydrogen evolution. Appl. Catal. B: Environ. 2021, 295, 120280.
3. Atomic design of metal-based catalysts for value-added conversion of small molecules

School of Chemical Engineering and Technology, Xi’an Jiaotong University
No.28 Xianning West Road, Xi’an, Shaanxi 710049, P.R. China | Copyright © 2023 Xu Zhao Group
